Answer:
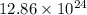 atoms of hydrogen
atoms of hydrogen
Step-by-step explanation:
The molecular formula for a water molecule is
 .
.
From the above formula, we can conclude that 1 molecule of water contains 2 atoms of hydrogen and 1 atom of oxygen.
There are
 molecules of water.
molecules of water.
1 molecule of water = 2 atoms of hydrogen.
∴
 molecules of water =
molecules of water =
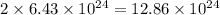 atoms of hydrogen.
atoms of hydrogen.
Therefore, there are
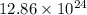 atoms of hydrogen in
atoms of hydrogen in
 molecules of water.
molecules of water.