Answer:
The energy of the photon is
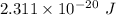 .
.
Step-by-step explanation:
Given:
The wavelength of the photon is given as:
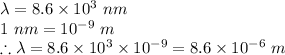
The energy of a photon in terms of its wavelength is given as:
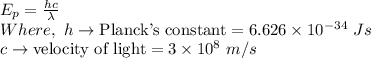
Plug in all the given values and calculate energy of the photon,
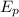 . This gives,
. This gives,
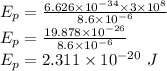
Therefore, the energy of the photon is
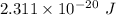 .
.