Step-by-step explanation:
a) The balance the chemical equation for the combustion reaction.
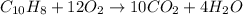
b) The amount of heat evolved on combustion of naphthalene = Q = -25.79 kJ
Mass of naphthalene = 0.6410 g
Moles of naphthalene =
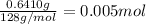
0.005 moles of naphthalene gives 25.79 kilo joules of heat. then 1 mol will give:
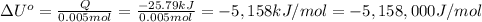
The standard change in internal energy (ΔU°) for the combustion of 1.000 mol naphthalene is -5,158 kJ/mol.
c)
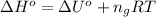
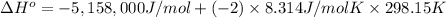
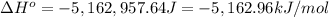
The standard enthalpy change (ΔH°) for the same reaction is -5,162.96 kJ/mol.
d) Standard enthalpy of formation per mole of naphthalene,
Enthlapy of formation of carbon dioxide ,
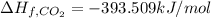
Enthlapy of formation of water ,
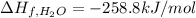
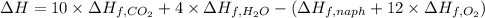
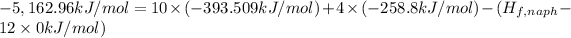
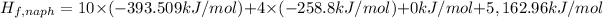
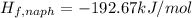
The standard enthalpy of formation per mole of naphthalene is 192.67 kJ/mol.