Answer:
change in volume of ballon is 2.109 L
Step-by-step explanation:
Given data:
initial temperature = 199 degree C = 292 K
pressure P1 = 1.09 atm
initial volume = 1.00 L
pressure P2 = 222 torr = 0.292 atm
temperature T2 = - 28 oC = 245 K
we know that
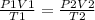
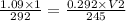
V2 = 3.109 L
change in volume of ballon = 3.109 - 1 = 2.109 L