Answer:
It is enough
Step-by-step explanation:
To develop the problem it is necessary to take into account the concepts related to the coefficient of performance of a pump.
The two ways in which the performance coefficient can be expressed are given by:
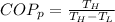
Where,
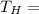 High Temperature
High Temperature
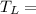 Low Temperature
Low Temperature
And the other way is,
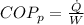
Where
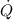 is heat rate and W the power consumed.
is heat rate and W the power consumed.
We have all our terms in Celsius, so we calculate the temperature in Kelvin
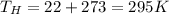
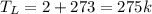
The rate at which heat is lost is:
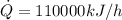
The power consumed by the heat pump is
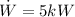
And the coefficient of performance is
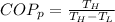
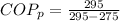
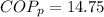
With this value we can calculate the Power required,
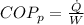
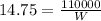
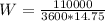
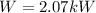
The power consumed is consumed is 5kW which is more than 2.07kW so this heat pump powerful enough.