Step-by-step explanation:
The given data is as follows.
Amount of racemic mixture = 8 g
Amount of pure R compound = 10 g
Therefore, total amount of R compound after missing is calculated as follows.
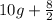
= 14 g
Let us assume that the amount of Si isomer is 4 g.
So, [R] = 11.5 g/L
[S] = 4 g/L
Now, we will calculate the enantiomeric excess as follows.
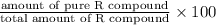
=
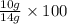
= 71.4%
Therefore, optical purity of the compound is 71.4%.
Now, the specific rotation will be calculated as follows.
Optical purity =
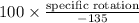
71.4% =
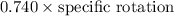
=
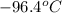
Therefore, specific rotation of the resulting mixture of the compound is
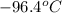 .
.