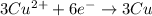Answer : The balanced reduction half-reaction is:
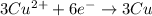
Explanation :
Redox reaction or Oxidation-reduction reaction : It is defined as the reaction in which the oxidation and reduction reaction takes place simultaneously.
Oxidation reaction : It is defined as the reaction in which a substance looses its electrons. In this, oxidation state of an element increases. Or we can say that in oxidation, the loss of electrons takes place.
Reduction reaction : It is defined as the reaction in which a substance gains electrons. In this, oxidation state of an element decreases. Or we can say that in reduction, the gain of electrons takes place.
The given balanced redox reaction is :

The half oxidation-reduction reactions are:
Oxidation reaction :
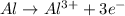
Reduction reaction :
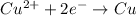
In order to balance the electrons, we multiply the oxidation reaction by 2 and reduction reaction by 3 and then added both equation, we get the balanced redox reaction.
Oxidation reaction :
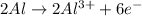
Reduction reaction :
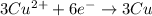
The balanced redox reaction will be:
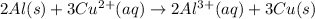
Thus, the balanced reduction half-reaction is: