Answer:
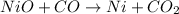 : reducing agent is CO.
: reducing agent is CO.
Step-by-step explanation:
a)
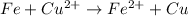
The oxidation state of copper reduces from +2 to 0, it is getting reduced. As
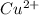 gets reduced, it acts as an oxidising agent.
gets reduced, it acts as an oxidising agent.
The oxidation state of iron increases from 0 to +2. Thus, it is getting oxidized. As
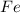 gets oxidized, it acts as a reducing agent.
gets oxidized, it acts as a reducing agent.
b.
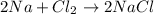
The oxidation state of chlorine reduces from 0 to -1, it is getting reduced. As
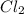 gets reduced, it acts as an oxidising agent.
gets reduced, it acts as an oxidising agent.
The oxidation state of sodium increases from 0 to +1. Thus, it is getting oxidized. As
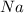 gets oxidized, it acts as a reducing agent.
gets oxidized, it acts as a reducing agent.
c.
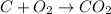
The oxidation state of oxygen reduces from 0 to -2, it is getting reduced. As
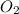 gets reduced, it acts as an oxidising agent.
gets reduced, it acts as an oxidising agent.
The oxidation state of carbon increases from 0 to +2. Thus, it is getting oxidized. As
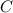 gets oxidized, it acts as a reducing agent.
gets oxidized, it acts as a reducing agent.
d.
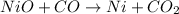
The oxidation state of nickel reduces from +2 to 0, it is getting reduced. As
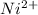 gets reduced, it acts as an oxidising agent.
gets reduced, it acts as an oxidising agent.
The oxidation state of carbon increases from +2 to +4. Thus, it is getting oxidized. As
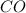 gets oxidized, it acts as a reducing agent.
gets oxidized, it acts as a reducing agent.