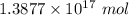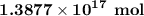
Explanation:
We are considering
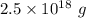 of water.
of water.
We know that each mole of a substance weights equal to the substances' molar weight.
The molar weight of water (
 ) is
) is
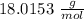 . This value is a standard and hence can be found from charts.
. This value is a standard and hence can be found from charts.
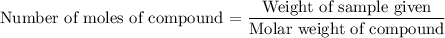
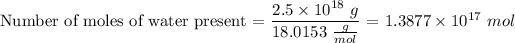
∴ Number of moles of water =