Answer: D. 19.9 g hydrogen remains.
Step-by-step explanation:
To calculate the moles, we use the equation:
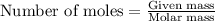
a) moles of

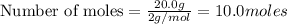
b) moles of
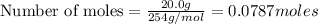
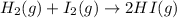
According to stoichiometry :
1 mole of
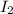 require 1 mole of
require 1 mole of

Thus 0.0787 moles of
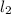 require=
require=
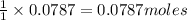 of
of

Thus
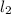 is the limiting reagent as it limits the formation of product and
is the limiting reagent as it limits the formation of product and
 acts as the excess reagent. (10.0-0.0787)= 9.92 moles of
acts as the excess reagent. (10.0-0.0787)= 9.92 moles of
 are left unreacted.
are left unreacted.
Mass of
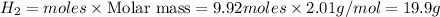
Thus 19.9 g of
 remains unreacted.
remains unreacted.