Answer:

Step-by-step explanation:
Hello,
This is a typical case in which the following chemical reaction is carried out:
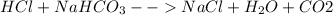
Since 50 mL of a 0.091M solution of HCl is employed, we perform the shown below stoichiometric calculation to find the sodium hydrogen carbonate grams that must be ingested by the woman:
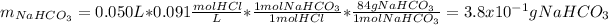
Best regards.