Answer:
A. The Salicylic acid
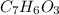 is the limiting reagent.
is the limiting reagent.
B. 4.66 g of the acetic anhydride
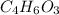 remain.
remain.
C. 2.6 g of
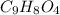 are produced.
are produced.
Step-by-step explanation:
A. First write the balanced chemical equation fior the asprin synthesis:
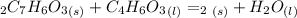
Then determine the limiting reagent.
To determine the limiting reagent first divide the mass of each compound between its molar mass, then divide this quantity by the number of moles given by the reaction and the smallest number will be the limiting reagent.
- For the Salicylic acid
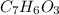 :
:
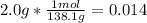
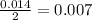
- For the Acetic anhydride
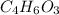 :
:
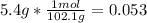
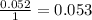
The smallest number is for the salicylic acid therefore it is the limiting reagent.
B. Calculate how many grams of the excess reagent remain.
- First calculate how many grams of acetic anhydride reacts:
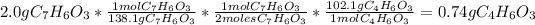 reacts
reacts
- Then subtract the quantity of acetic anhydride that reacts from the quantity that are mixed:
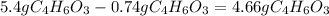 remain
remain
C. Calculate how many grams of product are produced:
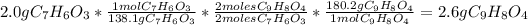 are produced.
are produced.