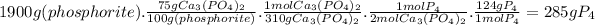Answer:
285 g of P₄
Step-by-step explanation:
Let's consider the following balanced equation.
2 Ca₃(PO₄)₂ + 6 SiO₂ + 10 C → 6 CaSiO₃ + P₄ + 10 CO
We know the following relations:
- 100 g of phosphorite contain 75 g of Ca₃(PO₄)₂
- 2 moles of Ca₃(PO₄)₂ produce 1 mole of P₄
- The molar mass of Ca₃(PO₄)₂ is 310 g/mol
- The molar mass of P₄ is 124 g/mol
Then, for 1.9 kg of phosphorite: