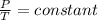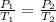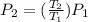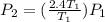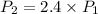Answer:
P(final) is 2.4 times P(initial).
Step-by-step explanation:
Here we can assume that the cylinder did not break and it's volume and number of moles of gas present in the cylinder remains constant.
Given the temperature increases by a factor of 2.4. Let us assume that the initial temperature be
 and the final temperature be
and the final temperature be
 .
.
Given that
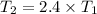
Now we know the ideal gas equation is PV=nRT
here V=constant , n=constant , R=gas constant(which is constant).