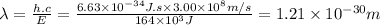Answer:
1.21 × 10⁻³⁰ m
Step-by-step explanation:
164 kJ of energy (E) are required to change the conformation of 1 mole of 11-cis-retinal. The wavelength (λ) of the radiation having such energy can be calculated through the Planck-Einstein equation.
E = h . ν = h . c . λ⁻¹
where,
h is the Planck constant
ν is the frequency
c is the speed of light
λ is the wavelength