Answer:
3moles
Step-by-step explanation:
Given that Volume of the vessel (V)=8L
Pressure of the vessel (P)=9 atm
Temperature of the vessel (T)=20°C=293K (∵K=273+°C )
Now we know that every ideal gas follows
PV=nRT
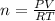
We know that R=0.082057

Now
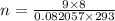
n=3moles