Answer:
22.48°C is the final temperature of the solution.
Step-by-step explanation:
Heat of neutralization of reaction , when 1 mol of nitric acid reacts= ΔH= -56.2 kJ/mol= -56200 J/mol
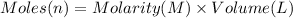
Moles of nitric acid = n
Volume of nitric acid solution =
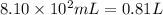
Molarity of the nitric acid = 0.600 M
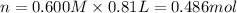
Moles of barium hydroxide = n'
Volume of barium hydroxide solution =
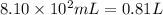
Molarity of the barium hydroxide= 0.300 M
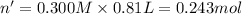
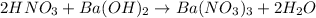
According to reaction, 2 mol of nitric acid reacts with 1 mol of barium hydroxide .Then 0.486 mol of nitric acid will react with :
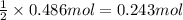 barium hydroxide.
barium hydroxide.
Heat release when 0.486 mol of nitric acid reacted = Q
= ΔH × 0.486 = -56200 J/mol × 0.486 mol=-27,313.2 J
Heat absorbed by the mixture after reaction = Q' = -Q = 27,313.2 J
Volume of the nitric solution = 0.81 L = 810 mL
Volume of the ferric nitrate solution = 0.81 L = 810 mL
Total volume of the solution = 810 mL + 810 mL = 1620 mL
Mass of the final solution = m
Density of water = density of the final solution = d = 1 g/mL
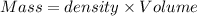
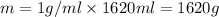
Initial temperature of the both solution were same =
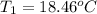
Final temperature of the both solution will also be same after mixing=

Heat capacity of the mixture = c = 4.184 J/g°C
Change in temperature of the mixture = ΔT =
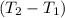
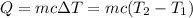

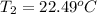
22.48°C is the final temperature of the solution.