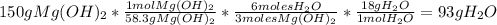Answer:
93 g of
 are needed to produce 150 g of
are needed to produce 150 g of
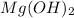
Step-by-step explanation:
1. Writhe the balanced equation given by the problem:
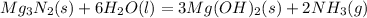
2. Then use the stoichiometry of the reaction to calculate the mass of
 that is needed to produce 150g of
that is needed to produce 150g of
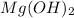 :
:
It is important to take in account the molar mass of the
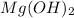 and the
and the
 for the calculations.
for the calculations.
Molar mass of the
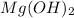 = 58.3g
= 58.3g
Molar mass of the
 = 18g
= 18g