Answer:
(3S)-2-chloro-2,3-dimethylpentane is produced exclusively.
Step-by-step explanation:
Electrophilic addition to (3S)-2,3-dimethylpent-1-ene proceeds through a carbocationic intermediate.
In the first step,
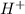 adds onto double bond to produce more stable tertiary carbocation. (protonation)
adds onto double bond to produce more stable tertiary carbocation. (protonation)
In the second step,
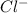 adds onto carbocation to produce (3S)-2-chloro-2,3-dimethylpentane exclusively.(nucleophilic addition)
adds onto carbocation to produce (3S)-2-chloro-2,3-dimethylpentane exclusively.(nucleophilic addition)
So, option (d) is correct.