Answer:
720.535 K
Step-by-step explanation:
Concept tested: Combined gas law
From the question we are given;
- Initial volume, V1 of the gas is 285 mL
- Initial pressure, P1 of the gas is 1.88 atm
- Initial temperature, T1 of gas is 355 K
- Final pressure of the gas, P2 = 2.50 atm
- Final volume of the gas, V2 = 435 mL
We are required to calculate the new temperature, T2of the gas;
- To solve the question we are going to use the combined gas law.
- According to the combined gas law;
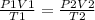
- Rearranging the formula we can calculate the new temperature;
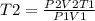
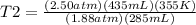
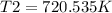
Therefore, the new temperature of the gas in the syringe must be 720.535 K