Step-by-step explanation:
Let us assume that the molar concentrations of tryptophan and tyrosine be x and y respectively.
Mathematically, A =
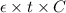
where, A = absorbance
 = molar absorption coefficient
= molar absorption coefficient
t = thickness of the cell
C = molar concentration
So, first calculate the molar concentration of tryptophan at 240 nm as follows.
0.66 =
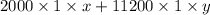
x =
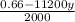 ........... (1)
........... (1)
At 280 nm,
0.221 =
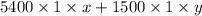
0.221 =
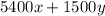 ........... (2)
........... (2)
Now, we will substitute the value of x from equation (1) into equation (2) as follows.
0.221 =
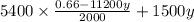
0.221 = 1.782 - 30240y + 1500y
0.221 = 1.782 - 28740y
28740y = 1.561
y =
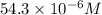
or, = 54.3
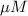 ............ (3)
............ (3)
Hence, the molar concentration of tyrosine is 54.3
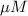 and putting this value into equation (1) we will get the value for concentration of tryptophan as follows.
and putting this value into equation (1) we will get the value for concentration of tryptophan as follows.
x =
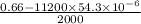
=
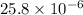
or, = 25.8
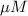
Therefore, we can conclude that the concentration of tryptophan is 25.8
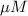 and concentration of tyrosine is 54.3
and concentration of tyrosine is 54.3
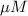 .
.