Answer : The correct option is, (E) 7.8 atm
Explanation :
The partial pressure of
 = 8.00 atm
= 8.00 atm
The partial pressure of
 = 5.00 atm
= 5.00 atm
 = 0.0025
= 0.0025
The balanced equilibrium reaction is,
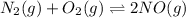
Initial pressure 8.00 5.00 0
At eqm. (8.00-x) (5.00-x) 2x
The expression of equilibrium constant
 for the reaction will be:
for the reaction will be:
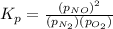
Now put all the values in this expression, we get :
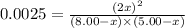
By solving the terms, we get:
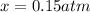
The equilibrium partial pressure of
 = (8.00 - x) = (8.00 - 0.15) = 7.8 atm
= (8.00 - x) = (8.00 - 0.15) = 7.8 atm
Therefore, the equilibrium partial pressure of
 is 7.8 atm.
is 7.8 atm.