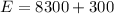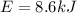Answer:
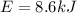
Step-by-step explanation:
By energy conservation we know that
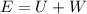
i.e. energy supplied by the pump is sum of the work done and increase in its internal energy
So here we know that
work done to pull the water from the well is given as
W = 8.3 kJ
also the increase in its internal energy is given as
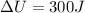
so total energy required is given as