Answer: The equilibrium molarity of oxygen gas is 7.1 M
Step-by-step explanation:
We are given:
Moles of oxygen gas = 1.3 mole
Moles of NO = 1.5 moles
Volume of the flask = 250 mL = 0.250 L (Conversion factor: 1 L = 1000 mL)
To calculate the molarity, we use the equation:
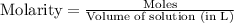
Molarity of oxygen gas =

Molarity of NO =
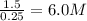
The chemical equation follows:
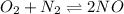
Initial: 5.2 - 6.0
At eqllm: 5.2+x +x 6.0-2x
The expression of
 for above equation follows:
for above equation follows:
![K_c=([NO])/([O_2][N_2])](https://img.qammunity.org/2020/formulas/chemistry/college/xo24zkx4gqixbyaaos5jbtbz17h3l0pjcn.png)
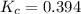 (Assuming)
(Assuming)
Putting values in above equation, we get:
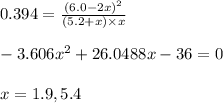
Neglecting the value of x = 5.4 because this cannot be greater than the initial value.
Concentration of oxygen gas at equilibrium = (5.2 + x) = 5.2 + 1.9 = 7.1 M
Hence, the equilibrium molarity of oxygen gas is 7.1 M