Step-by-step explanation:
A) Pressure at which HPLC procedure is running = P =
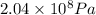
1 Torr = 133.322 Pascal
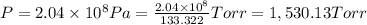
The running pressure in Torr is 1,530.13.
B)Initial temperature if the gas in balloon =
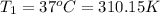
Initial volume of the gas in the balloon =
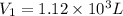
Final temperature if the gas in balloon =
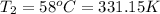
Final volume of the gas in the balloon =
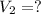
Using Charles law:
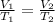 (constant pressure)
(constant pressure)
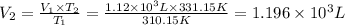
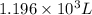 is the new volume of the gas.
is the new volume of the gas.
C) Initial temperature if the gas in balloon =
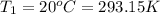
Initial volume of the gas in the balloon =
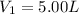
Initial pressure of the gas in the balloon =
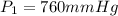
Final temperature if the gas in balloon =
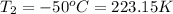
Final volume of the gas in the balloon =
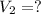
Final pressure of the gas in the balloon =
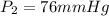
Using combine gas law:
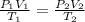
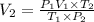
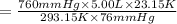
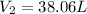
38.06 liters is the new volume of the balloon.
D) Initial temperature if the gas in container=
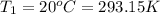
Initial volume of the gas in the container =
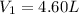
Initial pressure of the gas in the container=
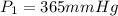
Final temperature if the gas in container=
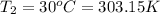
Final volume of the gas in the container=
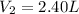
Final pressure of the gas in the container=
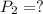
Using combine gas law:
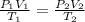
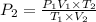
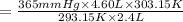
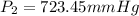
723.45 mmHg is the new pressure inside the container.