Step-by-step explanation:
Let us assume that the given data is as follows.
mass of water = 8.10 kg = 8100 g (as 1 kg = 1000 g)
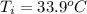 = (33.9 + 273) K = 306.9 K
= (33.9 + 273) K = 306.9 K
 = ?
= ?
q = 69.0 kJ = 69000 J (as 1 kJ = 1000 J)
As the relation between heat energy, specific heat and temperature change is as follows.
Q =
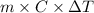
Now, putting the given values into the above formula as follows.
Q =
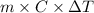
69000 =
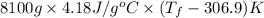
 = 309 K
= 309 K
Thus, we can conclude that the new temperature of the water bath is 309 K.