Answer: The enthalpy of the formation of
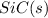 is coming out to be -65.3 kJ/mol
is coming out to be -65.3 kJ/mol
Step-by-step explanation:
Enthalpy change is defined as the difference in enthalpies of all the product and the reactants each multiplied with their respective number of moles. It is represented as
The equation used to calculate enthalpy change is of a reaction is:
![\Delta H^o_(rxn)=\sum [n* \Delta H^o_f_((product))]-\sum [n* \Delta H^o_f_((reactant))]](https://img.qammunity.org/2020/formulas/chemistry/high-school/5c7vs79qepq19tdktywtbtker28hpuplvy.png)
For the given chemical reaction:
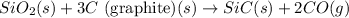
The equation for the enthalpy change of the above reaction is:
![\Delta H^o_(rxn)=[(1* \Delta H^o_f_((SiC(s))))+(2* \Delta H^o_f_((CO(g))))]-[(1* \Delta H^o_f_((SiO_2(s))))+(3* \Delta H^o_f_((C(s))))]](https://img.qammunity.org/2020/formulas/chemistry/college/d1iabhzc15x1y6bcmgkfvggp09y42qxlx9.png)
We are given:
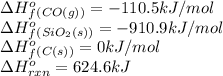
Putting values in above equation, we get:
![624.6=[(1* \Delta H^o_f_((SiC(s))))+(2* (-110.5))]-[(1* (-910.9))+(3* (0))]\\\\\Delta H^o_f_((SiC(s)))=-65.3kJ/mol](https://img.qammunity.org/2020/formulas/chemistry/college/krg0h32kap8uoxw01zctxrinjka8tjuijj.png)
Hence, the enthalpy of the formation of
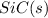 is coming out to be -65.3 kJ/mol.
is coming out to be -65.3 kJ/mol.