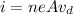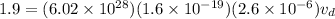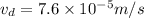Answer:
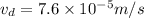
Step-by-step explanation:
As we know that electric current is given as
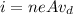
here we know that
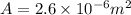
i = 1.9 A

Molar mass of the wire is
M = 26.98 g/mol
density of the wire is
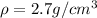
now let the mass of the wire is M gram
so number of atoms of Al in the wire is given as
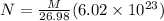
now number density is given as
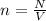
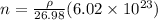
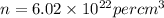
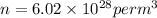
now we will have