Answer:
Mass of excess reactant leftover after reaction is complete = 34.2 g
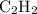 .
.
Step-by-step explanation:
Acetylene reacts with oxygen to form carbon dioxide and water, equation given is unbalanced
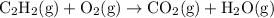
Firstly equation is balanced:
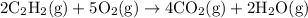
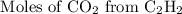
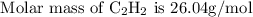
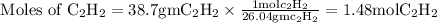
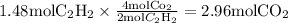
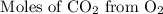 :
:
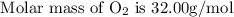
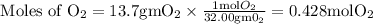
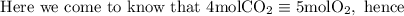
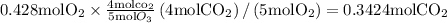
Here we come to know O2 is limiting reactant as it gives smaller amount of
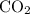 So
So
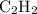 is excess reactant.
is excess reactant.
Mass of excess reactant utilized:
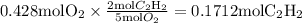
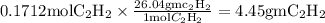
Mass of excess reactant leftover after reaction is complete:
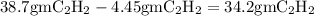 .
.