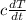Answer:
The required relation is,
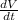 =
=
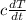
Explanation:
We know that for a certain amount of dry gas when the pressure is kept constant it's volume V and temperature T is related by the function,
V = cT -----------------------(1) [where "c" is a constant]
So, in that case rate of change of volume(V) with respect to time (t),
=
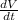
will be equal to ,
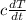
where
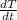 is equal to rate of change of temperature (T) with respect to time (t) and c is the constant stated before.
is equal to rate of change of temperature (T) with respect to time (t) and c is the constant stated before.
So, the required relation is,
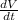 =
=