Answer:
The freezing point of a 1.7 mole aqueous ethylene glycol solution is 3.2°C
Step-by-step explanation:
Moles present in aqueous ethylene glycol solution= m=1.7
Depression constant for water =
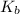 = 1.8 deg C/m
= 1.8 deg C/m
According to formula
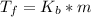
 =1.8* 1.7
=1.8* 1.7
=3.2
Hence the freezing point of aqueous ethylene glycol solution is 3.2°C