Answer : The chemical species X, Y, and Z in the mechanism are
 ,
,
 and
and
 respectively.
respectively.
Explanation :
The given overall balanced reaction is:
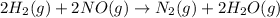
The two intermediate reactions are:
(1)
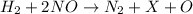
(2)
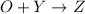
In reaction 1, there are 2 hydrogen atoms present on left side of reaction but there is no hydrogen atom present on right side of the reaction and there are 2 oxygen atoms present on left side of reaction but there is 1 oxygen atom present on right side of the reaction.
So, in order to balance the chemical reaction we are adding water molecule
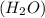 on right side of the reaction. So, the reaction 1 will be:
on right side of the reaction. So, the reaction 1 will be:
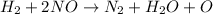
Thus, the chemical specie X will be

Now comparing the reaction 1 and the overall reaction we conclude that there are 2 molecules of
 present on right side of the reaction.
present on right side of the reaction.
So, in order to balance the number of water molecules we are taking the chemical specie Z as
 and to complete the reaction one
and to complete the reaction one
 molecule are adding on the left side of the reaction 2. So, the reaction 2 will be:
molecule are adding on the left side of the reaction 2. So, the reaction 2 will be:
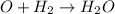
Thus, the chemical specie Z and Y will be
 and
and
 respectively.
respectively.