Answer:
Step-by-step explanation:
The reaction that takes place is
- NaH(s) + H₂O(l) → NaOH(aq) + H₂(g)
To find the mass of H₂ liberated we use PV=nRT:
- 755 mmHg ⇒755/760 = 0.993 atm
- 35°C ⇒ 35 + 273.16 = 308.16 K
- 0.993 atm * 0.475 L = n * 0.082atm·L·mol⁻¹·K⁻¹ * 308.16 K
- Mass H₂ = 0.01867 mol * 2g/mol = 0.03733 g
Then we use the moles of H₂ formed by the reaction to calculate the moles of NaH that reacted, and finally the mass using its molecular weight:
- 0.01867 mol H₂ *
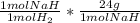 = 0.4480 g NaH
= 0.4480 g NaH