Answer:
Percent yield of carbon monoxide is approximately 38%.
Step-by-step explanation:
Given reaction : C + SiO₂ → Si + CO
On balancing it : 2C + SiO₂ → Si + 2CO
⇒ 2 mole of carbon produces 2 mole of carbon monoxide
or 2×12=24 g of carbon produces 2×(12+16)=56 g of carbon monoxide
⇒ Expected yield of carbon monoxide when 32.5g of carbon is heated with silicon dioxide is 32.5×
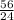
⇒ Expected yield =
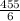 = 75.8 g
= 75.8 g
Actual yield is given as 28.8 g
⇒ The percent yield of carbon monoxide =
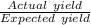 ×100
×100
=
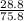 ×100
×100
≅38%