Answer:Ba

Step-by-step explanation:
option A:
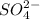 is an anion and
is an anion and
 is also an anion.So,there is no reaction.
is also an anion.So,there is no reaction.
option B:
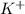 reacts with
reacts with
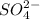 to form
to form
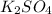 .
.
Since
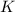 is a first group element,all its salts are soluble in water.
is a first group element,all its salts are soluble in water.
Hence no precipitate is formed.
option C:
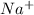 reacts with
reacts with
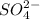 to form
to form
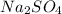 .
.
Since
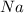 is a first group element,all its salts are soluble in water.
is a first group element,all its salts are soluble in water.
Hence no precipitate is formed.
option D:
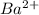 reacts with
reacts with
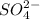 to form
to form
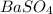 .
.
Solubility of sulphates of second group elements decrease down the group.
So,
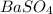 is a precipitate.
is a precipitate.