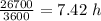Answer:
Diffusion time is 7.42 h
Solution:
As per the question:
Temperature, T =
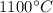
Surface concentration of arsenic,
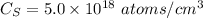
Surface concentration below Silicon surface,
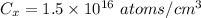
D =
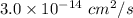
x =
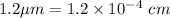
Initial concentration at t = 0,
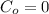
Now, by using Flick's second eqn:
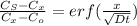
Thus by putting appropriate values:
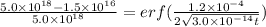
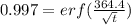 (1)
(1)
Now,
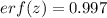
Now, from error function values tabulation:
For z = 2.0, erf(z) = 0.998
For z = 2.2, erf(z) = 0.995
Now,
With the help of linear interpolation method:
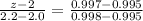
z = 2.12
Now, using eqn (1) and above value:
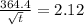
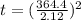 = 26700 s
= 26700 s
t =