Answer:
All of the statements are true
Step-by-step explanation:
Let's consider a reaction :

Molecular equation of this reaction should be written in terms of neutral and complete form of all compounds.
Molecular equation:

Complete ionic equation is written in terms of constituting ions of each compounds.
Complete ionic equation:
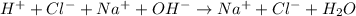
Same ions present in both side of complete ionic equation are omitted to write net ionic equation. These ions are called spectator ions.
Here
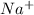 and Cl^{-} are spectator ions.
and Cl^{-} are spectator ions.
So, net ionic eqaution:
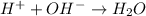
Hence all of the statements are true.