Answer:
Energy =
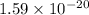 Joules
Joules
Step-by-step explanation:
We know,
Energy(E) carried by a wave =
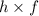
where,
h - Planck's constant =
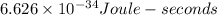
f - Frequency of the wave.
Given,
Frequency of given wave (f) =
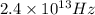
Therefore from the above formula,
Energy carried by wave =
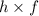
E =
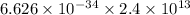
E =
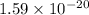 Joules
Joules
Therefore,
Energy carried by given wave =
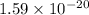 Joules
Joules