Answer : The molecular shape of
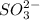 is trigonal pyramidal and bond angle between O-S-O is
is trigonal pyramidal and bond angle between O-S-O is
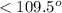
Explanation :
Lewis-dot structure : It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule.
In the Lewis-dot structure the valance electrons are shown by 'dot'.
The given molecule is,
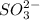
As we know that sulfur has '6' valence electrons and oxygen also has '6' valence electron.
Therefore, the total number of valence electrons in
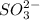 = 6 + 3(6) + 2 = 26
= 6 + 3(6) + 2 = 26
According to Lewis-dot structure, there are 8 number of bonding electrons and 18 number of non-bonding electrons or lone pair electrons.
The Lewis-dot structure of
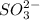 is shown below.
is shown below.
Formula used :
![\text{Number of electron pair}=(1)/(2)[V+N-C+A]](https://img.qammunity.org/2020/formulas/chemistry/college/oq9tu4xdhl4miobr3yimshosm0nub93rf0.png)
where,
V = number of valence electrons present in central atom
N = number of monovalent atoms bonded to central atom
C = charge of cation
A = charge of anion
Now we have to determine the hybridization of the given molecule,
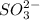
![\text{Number of electrons}=(1)/(2)* [6+2]=4](https://img.qammunity.org/2020/formulas/chemistry/college/hmtntubmosdoip0fz7qk7juttrdvcunmnb.png)
The number of electron pair are 4 that means the hybridization will be
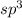 and the electronic geometry of the molecule will be tetrahedral.
and the electronic geometry of the molecule will be tetrahedral.
But as there are 3 atoms around the central sulfur atom, the fourth position will be occupied by lone pair of electrons. The repulsion between lone and bond pair of electrons is more and hence the molecular geometry will be trigonal pyramidal and the bond angle between O-S-O is less than
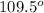 due to repulsion between lone and bond pair of electrons.
due to repulsion between lone and bond pair of electrons.