The names and symbols of elements that are divided into groups or families based on the chemical and physical properties are as follows.
Step-by-step explanation:
The arrangement of electrons in an atom is referred to as electronic configuration and valence electrons are electron number & structure present in last shell of atoms. There are 18 groups/families and 7 periods in periodic table. The elements which belong to each family based on their number of valence electrons as follows and here n is no of shell:
Alkali metal (1 valence electron /
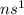 ): Name of the element: Lithium and Symbol: (Li) and Name of the element: Sodium and Symbol: (Na)
): Name of the element: Lithium and Symbol: (Li) and Name of the element: Sodium and Symbol: (Na)
Alkaline Earth Metal (2 valence electron /
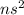 ): Name of the element: Beryllium and Symbol: (Be) & Name of the element: Magnesium and Symbol: (Mg)
): Name of the element: Beryllium and Symbol: (Be) & Name of the element: Magnesium and Symbol: (Mg)
Boron Family (3 valence electron /
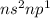 ): Name of the element: Boron and Symbol: (B) & Name of the element: Aluminum and Symbol: (Al)
): Name of the element: Boron and Symbol: (B) & Name of the element: Aluminum and Symbol: (Al)
Carbon Family (4 valence electron /
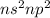 ): Name of the element: Carbon and Symbol: (C) & Name of the element: Silicon and Symbol: (Si)
): Name of the element: Carbon and Symbol: (C) & Name of the element: Silicon and Symbol: (Si)
Nitrogen Family (5 valence electron /
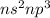 ): Name of the element: Nitrogen and Symbol: (N) & Name of the element: Phosphorus and Symbol: (P)
): Name of the element: Nitrogen and Symbol: (N) & Name of the element: Phosphorus and Symbol: (P)
Oxygen Family (6 valence electron /
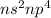 ): Name of the element: Oxygen and Symbol: (O) & Name of the element: Sulfur and Symbol: (S)
): Name of the element: Oxygen and Symbol: (O) & Name of the element: Sulfur and Symbol: (S)
Halogens Family (7 valence electron /
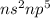 ): Name of the element: Fluorine and Symbol: (F) & Name of the element: Chlorine and Symbol: (Cl)
): Name of the element: Fluorine and Symbol: (F) & Name of the element: Chlorine and Symbol: (Cl)
Nobel Gases (8 valence electron /
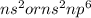 / Octet complete or complete outermost shell): Name of the element: Helium and Symbol: (He), Name of the element: Neon and Symbol: (Ne) and Name of the element: Argon and Symbol: (Ar)
/ Octet complete or complete outermost shell): Name of the element: Helium and Symbol: (He), Name of the element: Neon and Symbol: (Ne) and Name of the element: Argon and Symbol: (Ar)