Answer:
Reaction B has an enzyme.
Step-by-step explanation:
In both charts, we can see the energy versus the progress of the reaction. In both charts, reactants and products have the same energy, but chart B has a lower activation energy (The difference in energy between the reactants and the activated complex). This is probably due to the action of an enzyme. Enzymes lower the activation energy in order to increase the reaction rate as we can see in the Arrhenius equation.
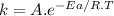
where,
k is the rate constant
A is the collision factor
Ea is the activation energy
R is the ideal gas constant
T is the absolute temperature
The higher the rate constant, the higher the reaction rate.