Answer:
pH = 12.7
Step-by-step explanation:
First, we have to calculate the [Ca²⁺] in a solution of about 250 ppm CaCO₃.
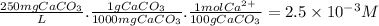
Now, let's consider the dissolution of Ca(OH)₂ in water.
Ca(OH)₂(s) ⇄ Ca²⁺(aq) + 2 OH⁻(aq)
The solubility product Ksp is:
Ksp = [Ca²⁺] × [OH⁻]²
[OH⁻] = √(Ksp/[Ca²⁺]) = √(6.5 × 10⁻⁶/2.5 × 10⁻³) = 5.1 × 10⁻² M
Finally, we can calculate pOH and pH.
pOH = -log [OH⁻] = -log (5.1 × 10⁻²) = 1.3
pH + pOH = 14 ⇒ pH = 14 - pOH = 14 - 1.3 = 12.7