Answer:
The product of Pressure and Volume is a constant.
Step-by-step explanation:
We know the Ideal gas equation,
PV = nRT,
where,
P - Pressure of the gas
V - Volume of the gas
n - No. of moles of the gas taken
R - Universal Gas Constant.
T - Temperature of the gas.
We know that , n =
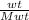
where, wt - Mass of gass ; Mwt - Molecular weight of the gas.
We are given the weight of the gas remains constant (wt is a constant)
Therefore as n =
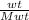 ,(where both wt and Mwt are constants)
,(where both wt and Mwt are constants)
n is a constant in this process.
Universal gas constant is a constant
Given temperature is a constant.
Therefore,
As, PV = nRT, (where n,R,T are constants),
PV is a constant.
Therefore we can say,
PV = k, for some constant k.
If we plot a graph for P vs V , we get a Rectangular Hyperbola( One of the general forms of a Rectangular Hyperbola is xy = c)