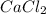 because it produces more moles of solute particles per mole of compound.
because it produces more moles of solute particles per mole of compound.
Step-by-step explanation:
Freezing point depression depend on the "amount of solute particles" . Freezing point is also a colligative property. As we know, calcium chloride will dissociate into 3 particles whereas sodium chloride dissociate into only 2 particles. So, an aqueous solution calcium chloride would be effective than sodium chloride.
Also the water's boiling point with calcium chloride is higher. So
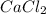 is considered as a good road salt since it produces more ions per formula unit of salt that is dissolved than NaCl
is considered as a good road salt since it produces more ions per formula unit of salt that is dissolved than NaCl