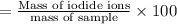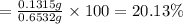Answer:
The mass% of iodide ions in the sample is 20.13%.
Step-by-step explanation:
Total Moles of silver nitrate added = n
Molarity of the silver nitrate solution = 0.05539 M
Volume of the silver nitrate = 50.00mL = 0.050 L
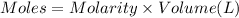
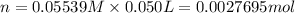
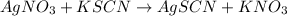 ..[1]
..[1]
Moles of potassium thiocyanate = n'
Molarity of the potassium thiocyanate solution = 0.05233 M
Volume of the potassium thiocyanate = 33.12 mL= 0.03312 L
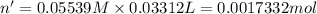
According to reaction-1, 1 mole of potassium thiocyanate reacts with 1 mol of silver nitrate. Then 0.0017332 mole of potassium thiocyanate reacts with 0.0017332 mol of silver nitrate.
Moles of silver nitrate which had reacted with iodide ions = N
n = n' + N
N = n - n' = 0.0027695 mol - 0.0017332 = 0.0010363 mol
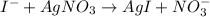 ..[2]
..[2]
According to reaction-2, 1 mole of iodide reacts with 1 mol of silver nitrate. Then 0.0010363 mole of silver nitrate reacts with :
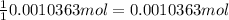 of silver nitrate.
of silver nitrate.
Mass of 0.0010363 mole of iodide ions =
0.0010363 mol × 126.9 g/mol = 0.1315 g
The mass% of iodide ions in the sample:
Mass of sample = 0.6532 g