Answer:
1) 0.825 grams is the mass of pure calcium carbonate product collected at the end of the experiment.
2) The mass % calcium carbonate in the tablet is 50.58%.
3) The mass % of calcium in calcium carbonate is 40.00 %.
4) Mass of calcium in a tablet is 0.33 g.
Step-by-step explanation:
Mass of tablet = 1.631 g
Mass of the watch glass = 46.719 g
Mass of the watch glass + mass of calcium carbonate = 47.544 g.
Mass of calcium carbonate = 47.544 g - 46.719 g = 0.825 g
0.825 grams is the mass of pure calcium carbonate product collected at the end of the experiment.
Mass of calcium carbonate = 0.825 g
Percentage of calcium in tablet:
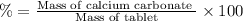
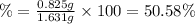
The mass % calcium carbonate in the tablet is 50.58%.
Percentage of calcium in calcium carbonate :
Molar mass of calcium carbonate = 100 g/mol
Atomic mass of calcium = 40 g/mol
Percentage of calcium in calcium carbonate :
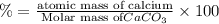
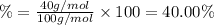
The mass % of calcium in calcium carbonate is 40.00 %.
Mass of calcium carbonate = 0.825 g
The mass % of calcium in calcium carbonate is 40.00 %.
Mass of calcium in a tablet : x
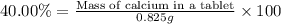
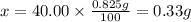
Mass of calcium in a tablet is 0.33 g.