Answer:
E₁ = 1.042 eV
E₄₋₃= 7.29 eV
E₄₋₂= 12.50 eV
E₄₋₁= 15.63 eV
E₃₋₂= 5.21eV
E₃₋₁= 8.34eV
E₂₋₁= 3.13eV
Step-by-step explanation:
The energy in an infinite square-well potential is giving by:
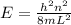
where, h: Planck constant = 6.62x10⁻³⁴J.s, n: is the energy state, m: mass of the electron and L: widht of the square-well potential
The energy of the electron in the ground state, n = 1, is:
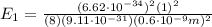
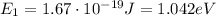
The photon energies that are emitted as the electron jumps to the ground state is the difference between the states:
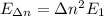
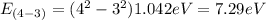
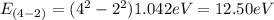
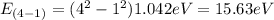
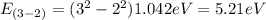
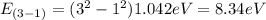
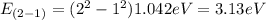
Have a nice day!