Answer:
a.) 1.453MW/m2, b.) 2,477,933.33 BTU/hr c.) 22,733.33 BTU/hr d.) 1,238,966.67 BTU/hr
Step-by-step explanation:
Heat flux is the rate at which thermal (heat) energy is transferred per unit surface area. It is measured in W/m2
Heat transfer(loss or gain) is unit of energy per unit time. It is measured in W or BTU/hr
1W = 3.41 BTU/hr
Given parameters:
thickness, t = 7.5mm = 7.5/1000 = 0.0075m
Temperatures 150 C = 150 + 273 = 423 K
50 C = 50 + 273 = 323 K
Temperature difference, T = 423 - 323 = 100 K
We are assuming steady heat flow;
a.) Heat flux, Q" = kT/t
K= thermal conductivity of the material
The thermal conductivity of brass, k = 109.0 W/m.K
Heat flux,
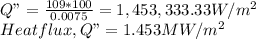
b.) Area of sheet, A = 0.5m2
Heat loss, Q = kAT/t
Heat loss,
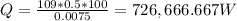
Heat loss, Q = 726,666.667 * 3.41 = 2,477,933.33 BTU/hr
c.) Material is now given as soda lime glass.
Thermal conductivity of soda lime glass, k is approximately 1W/m.K
Heat loss,
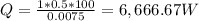
Heat loss, Q = 6,666.67 * 3.41 = 22,733.33 BTU/hr
d.) Thickness, t is given as 15mm = 15/1000 = 0.015m
Heat loss,
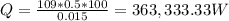
Heat loss, Q = 363,333.33 * 3.41 = 1,238,966.67 BTU/hr