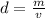Answer:
Density of the gas decreases.
Step-by-step explanation:
The relationship between the volume and temperature of a gas was described by Jack Charles, and it says that in a sample of gas at constant pressure it is observed that when the temperature is increased the volume of the gas also increases and that when the temperature decreases The volume also decreases.
Then, if you warm the balloon in a sunny window temperature will increase, therefore volume will increase.
According to density definition, if volume increase, density decreases.