Answer:
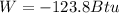
Step-by-step explanation:
Hello,
In this problem, the piston-cylinder assembly make us state the energy balance as:
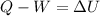
Thus, we must now compute
 in terms of Cv as follows:
in terms of Cv as follows:
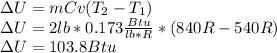
Now, since heat is given off, its sign is negative, thus, the work is computed as:
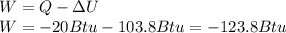
This work means that work was done over the system in order to allow the compression.
The suppositions were:
- The change in the internal energy is a function of the temperature.
- Air is an ideal gas.
Best regards.